Make Compliance Your Signature Move
Meet ZenSign, the compliant external eSignature tool from ZenQMS
Powered by ZenQMS, ZenSign empowers quality stakeholders to easily request and capture Part 11-compliant eSignatures from external clients, vendors, and more without ever leaving the ZenQMS platform.
Seamless eSignature integration = seamless compliance
Reduced validation and implementation burden.
You’ve already validated and implemented ZenQMS – no need to start the process from scratch. Our provided ZenSign validation materials make it easy to validate the additional feature and quickly put it to use.
Expand quality’s reach.
Anyone, anywhere can sign with just an email and a pincode. There’s no need for external vendors or clients to be users of the ZenQMS platform.
Quality documents never leave the system.
After verifying their identity, external signees receive a secure link to view and sign their assigned documents within the ZenQMS platform – no additional user account required. Documents never leave ZenQMS, keeping everything in a closed, secure workflow.
Serious security from day one.
X.509 certification is built into ZenSign and all signatures are Part 11-compliant from day one. Plus, every step is meticulously captured in a robust, unalterable audit trail, helping you meet regulatory requirements around the globe.
Effortless external collaboration.
Whether you’re collecting signatures for vendor agreements and other documents, or managing deviations and change controls, ZenSign handles it all.
Sign me up!
Want to see ZenSign in action and learn more about pricing? Let us know and we’ll reach out to connect!
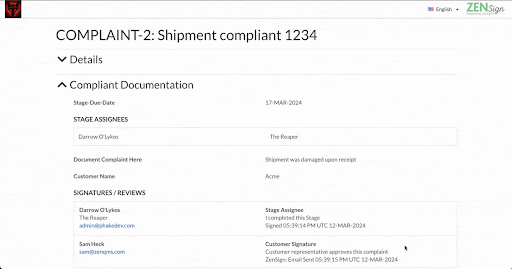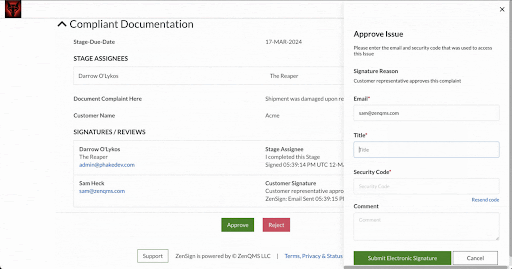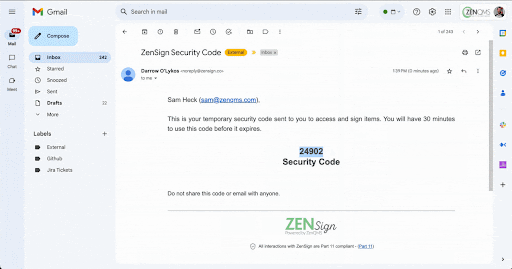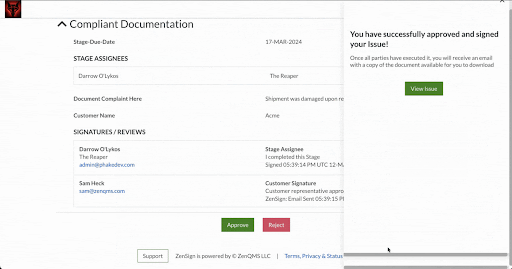
How does ZenSign work?
With ZenSign, you simply add an external party to your required signature approval workflow using their name and email address. The assigned party then receives an email with a link and pincode to verify their identity. Once verified, the assignee is able to review and sign the document within the ZenQMS platform, all without needing their own user account.
Do external signees need a ZenQMS account?
No, external signature assignees do not need to be added to your ZenQMS account.
Is ZenSign 21 CFR Part 11-compliant?
Yes. X.509 certification is built into ZenSign and all signatures are Part 11-compliant.
How much does ZenSign cost?
ZenSign has tier-based pricing based on the number of external signatures. The first 10 signatures are complimentary, allowing you to test out the feature and experience the benefits firsthand.
All Signs Point to ZenQMS
ZenSign is powered by ZenQMS, meaning you get the industry-leading support, quality expertise, and obsession with GxP and regulatory compliance our users know and trust.
