The flexible, all-in-one eQMS for Life Sciences & GxP-regulated companies
With ZenQMS, awesome comes standard.
- An integrated eQMS that teams actually like to use, with each module configured to mirror YOUR processes
- Instant access to all modules for all team members from day one
- We flipped the script with our pricing and support model, so your quality system can grow with your team
- Purpose-built for Life Sciences companies
- Validated for 21 CFR Pt.11/ Annex 11 and relevant GxP/ ISO requirements
Full access to the Quality functionality you need, from day one
ZenQMS gives you everything you need to manage Quality seamlessly. Whether you’re just getting started or a fully commercial GMP, ZenQMS allows you to create a single source of truth that is light-years beyond paper or paper on glass solutions.
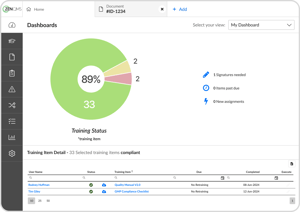
See training status at a glance with our simple dashboard
-
No more chasing down your team when training is due - users get automated reminders of new and past-due assignments
-
Easily create, complete, and track training from SOP reviews to on-the-job events
-
Pull training reports by individual, course, or group
-
Drive robust training and testing with SCORM file support
-
Access expert-authored GMP, GLP, and GCP training content integrated directly in the system
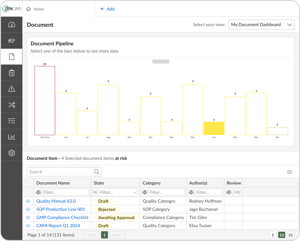
Track the document lifecycle from draft to retirement
-
Stay 21 CFR Part 11/ Annex 11 compliant for all electronic signatures
-
Edit documents collaboratively with other stakeholders
-
Establish document control through granular permissions
-
Create both major and minor document revisions
-
Search data, even within documents, using the Keyword Search feature
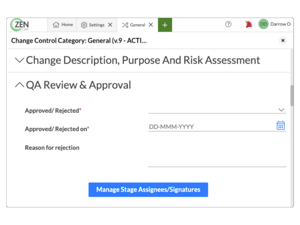
Manage organizational change in a controlled state
-
Keep an accurate record of change within your organization
-
Build follow-up tasks directly into your process so change is actionable
-
Assign detailed permissions for access and escalation
-
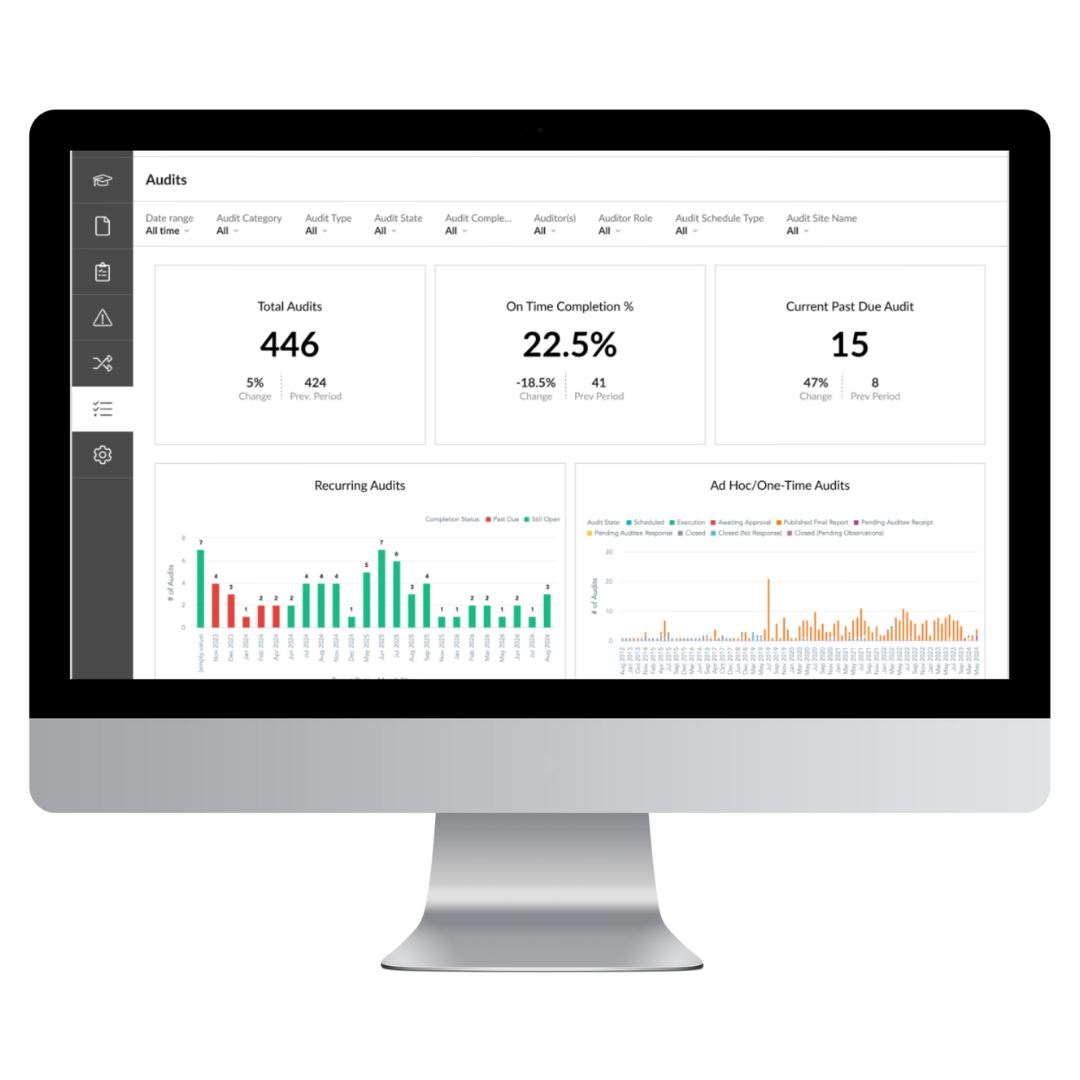
Reduce audit times with our straightforward interface that regulators understand
-
Automatically enforce closed-loop processes
-
Easily demonstrate your controlled state to auditors
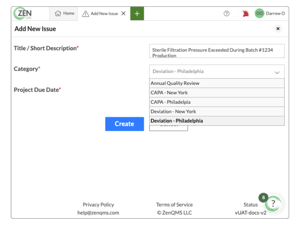
Report, address, and resolve issues without the headache
-
Handle CAPAs, deviations, and your quality control process with just a few clicks
-
Easily manage customer and product complaints all in one system
-
Build custom, closed-loop workflows that match your processes for any event type
-
Automate issue notifications and investigator assignments
-
Create dashboards to measure quality event progress and activity
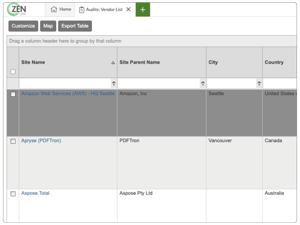
Simplify your entire GxP audit and supplier qualification process
-
Create and track lists of approved suppliers
-
Simplify supplier management with configurable Qsheets
-
Monitor observations to close
-
Set an evergreen audit calendar to update as you work
-
Establish a feedback loop for risk management with reports and trends
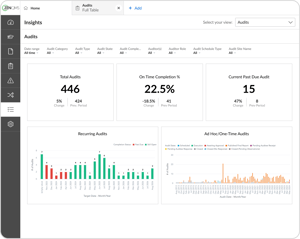
Get actionable insights from your quality data
-
Identify and analyze trend data on compliance, training, risks, and more
-
Provide leadership with invaluable insight to fuel improvement and growth
-
Visualize your custom KPIs with the Insights Pro
-
Export dashboards to PDF for easy sharing
-
Export data from visualizations to CSV and XLSX formats
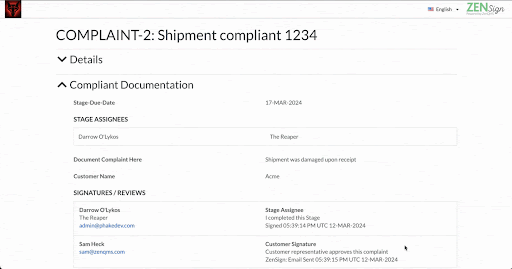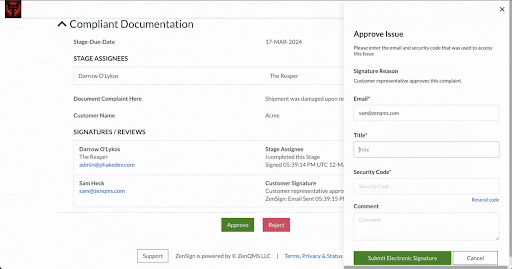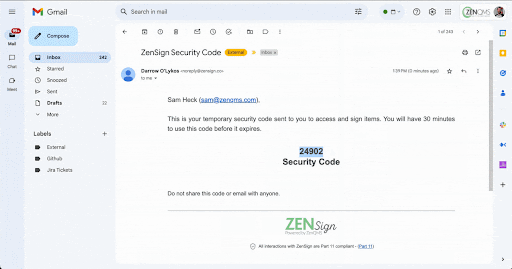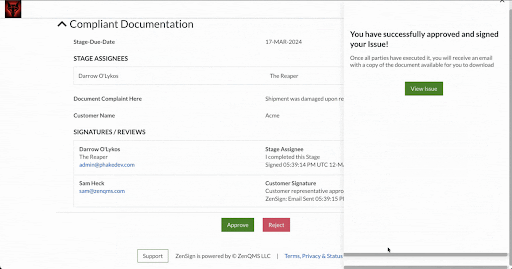
Make compliance your signature move. Have your external partners sign without DocuSign or Adobe Sign
-
Seamless Part-11 compliant eSignature to manage clients, vendors, and more
-
Already validated and integrated directly into ZenQMS
-
Secure closed workflow: documents and content never leave the system
-
X.509 certificate built in
Our customers are feeling Zen
Learn why ZenQMS is trusted by hundreds of life science’s most exciting and innovative companies from preclinical through commercialization and beyond.

Have peace of mind that we are partners on your quality journey.

100% Pure SaaS from birth
ZenQMS is built with a pure SaaS infrastructure from the ground up, which means that you always have automatic access to each new release with no IT requirements to manage.
Our single validated, multi-tenant instance in the cloud (Amazon Web Services) has a guaranteed uptime > 99.99% and expedited validation gameplan that anyone can execute.
Data Security and Transparency
ZenQMS maintains defined and tested procedures for business continuity and disruption. Your data gets backed up hourly and you have the ability to export your entire account locally as a zip file on a regular basis for added protection.
Quality is not optional, and we believe it should be everyone's business. So, we've flipped the model from complexity and cost to lifetime support and simple, transparent enterprise pricing.
Support should be personal, helpful, and free.
Support starts with personalized implementation, including a guided validation checklist, and continues for your lifetime at ZenQMS. Access to our helpdesk and ZenQMS training material (including our knowledge base) are all included as part of your annual fee--no surprises or hourly call fees.
Your voice and experience matter to us. We build our product roadmap in collaboration with our customers.
Our customers see a clear cost of ownership and no surprise charges for storage, support, seat licenses, etc.
When you invest in ZenQMS, you have access to the entire system for your whole company without exception. Rather than charging per module, all our modules and releases come standard.
Worrying about seat licenses means that you have to pay for growth. We believe that an eQMS should be able to grow with you and quality shouldn't be silo'd, so we don’t charge for seat licenses. Let's grow together.
